
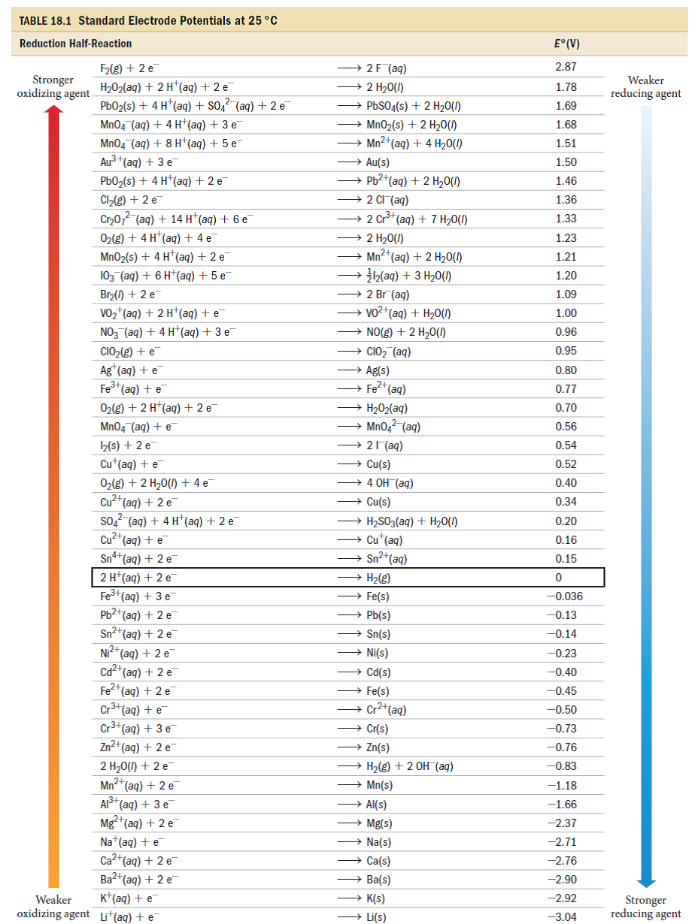
Chemistry Notes | Chemistry Pdf -- Electrochemistry and Galvanic Cells | Electrochemistry, Chemistry basics, Galvanic cell

Reduction Table.pdf - STANDARD REDUCTION POTENTIALS o(Volts) COUPLE - HF (H+) F2 - F- S2O82- - SO42- BiO3- - Bi3+ H2O2 - H2O (H+) PbO2 - PbSO4 (H+, | Course Hero

Chemistry Notes | Chemistry Pdf -- Electrochemistry and Galvanic Cells | Electrochemistry, Chemistry basics, Galvanic cell

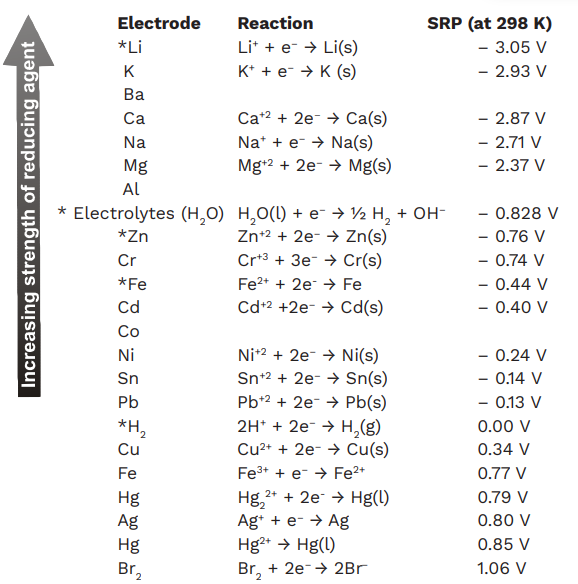
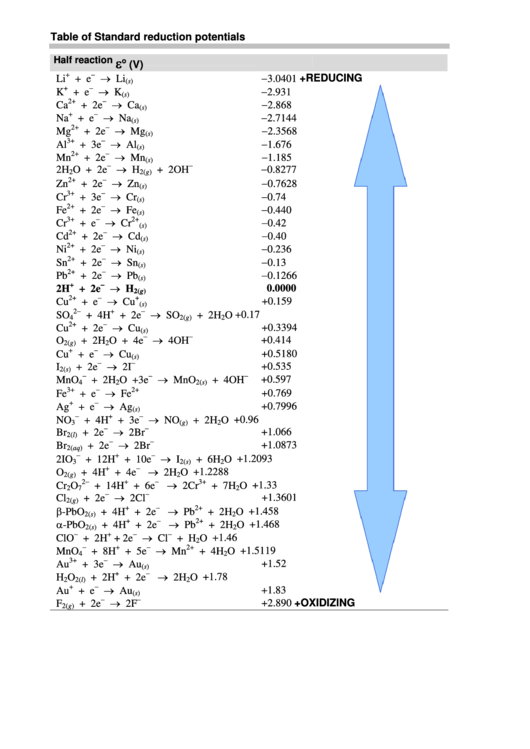
Table of Standard reduction potentials.pdf - Table of Standard reduction potentials Half reaction + Li + e Li(s) K+ + e K(s) Ca2+ + 2e Ca(s) Na+ + e | Course Hero
![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)





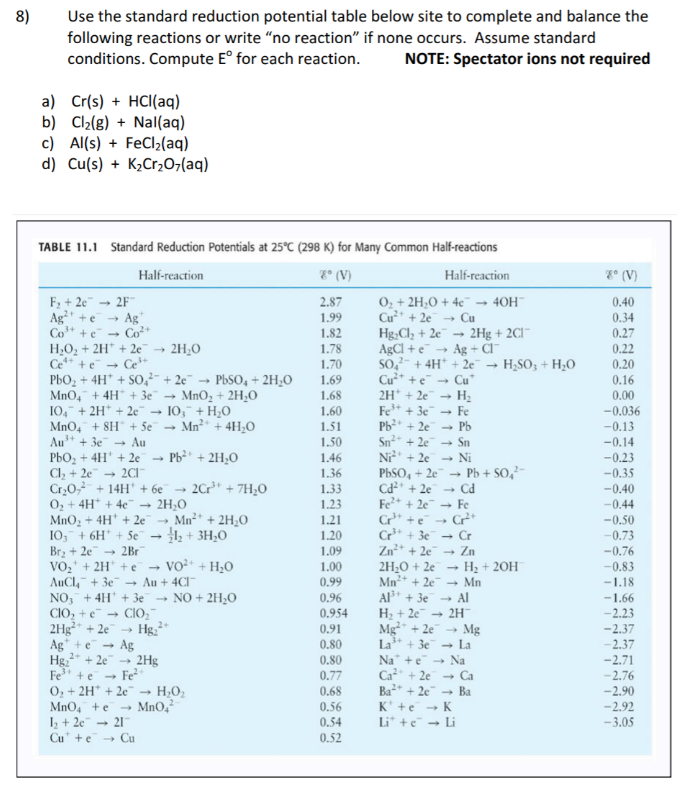
![PDF] ELECTROCHEMICAL SERIES | Semantic Scholar PDF] ELECTROCHEMICAL SERIES | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d69fb8901fa978ef52cc489d4cac71bb23da9922/1-Table1-1.png)





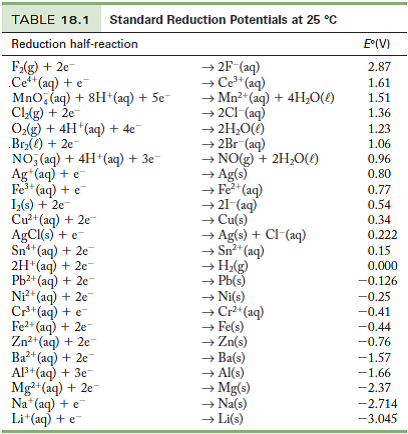
![Standard reduction potentials at 298°K. [24] | Download Table Standard reduction potentials at 298°K. [24] | Download Table](https://www.researchgate.net/publication/316026333/figure/tbl2/AS:650784626708491@1532170554986/Standard-reduction-potentials-at-298K-24.png)